Majumdar Lab
Center for Clinical Pharmacology
Majumdar Lab
Center for Clinical Pharmacology
Majumdar Lab
Research Projects
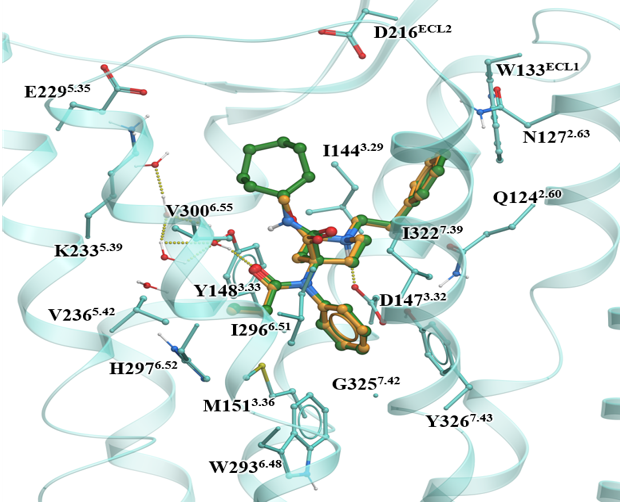
Using a structure-based design approach and a collaborative effort between Kobilka-Skiniotis-Katritch and McLaughlin groups, we recently reported the targeting of the sodium binding site in the mu opioid receptor. We utilized the fentanyl template and a bitopic approach to design a ligand C6 guano which shows analgesia but lower adverse effects in mice compared to canonical opioids.
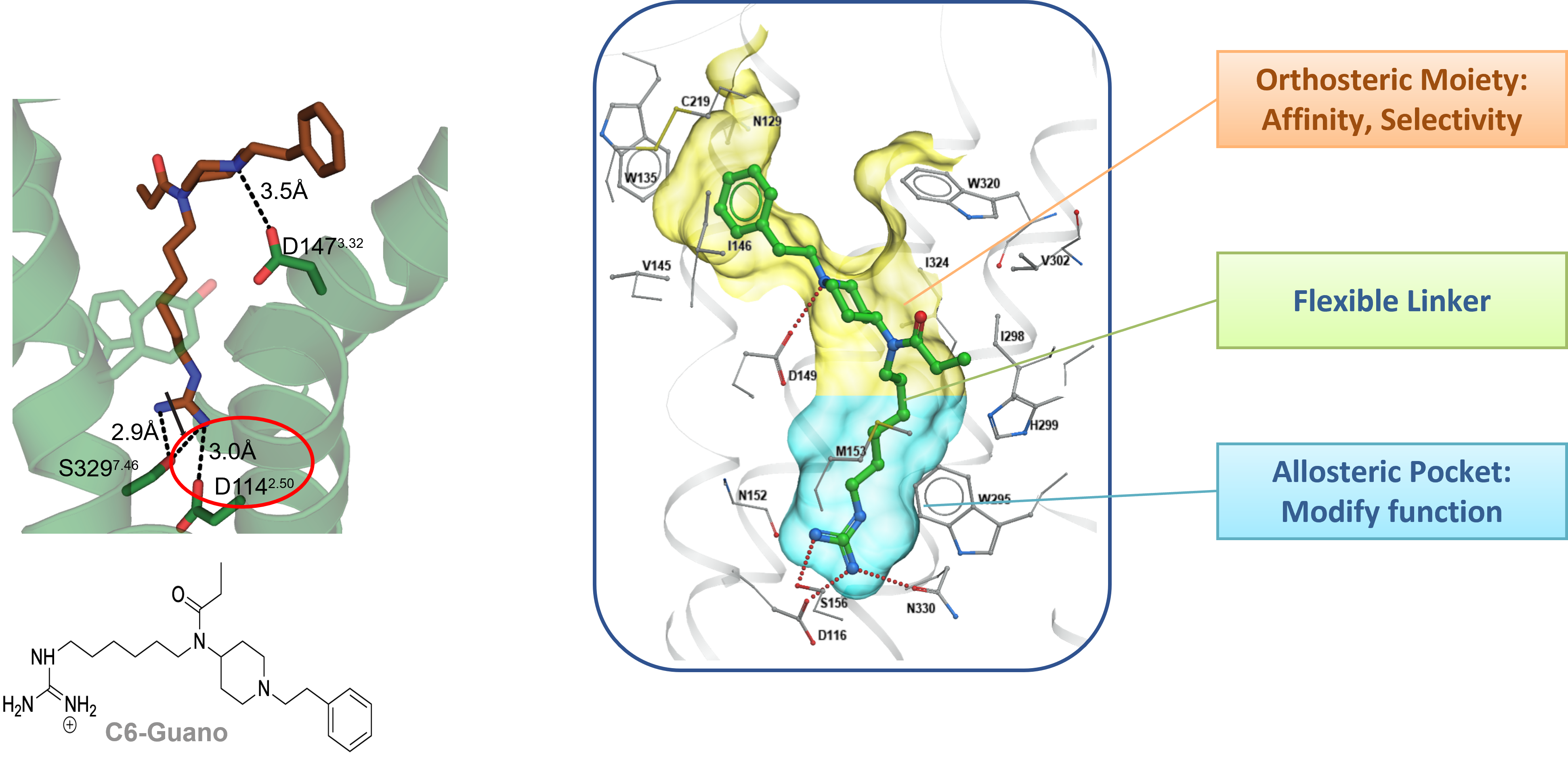
Nature 2023; 613 (7945):767-774
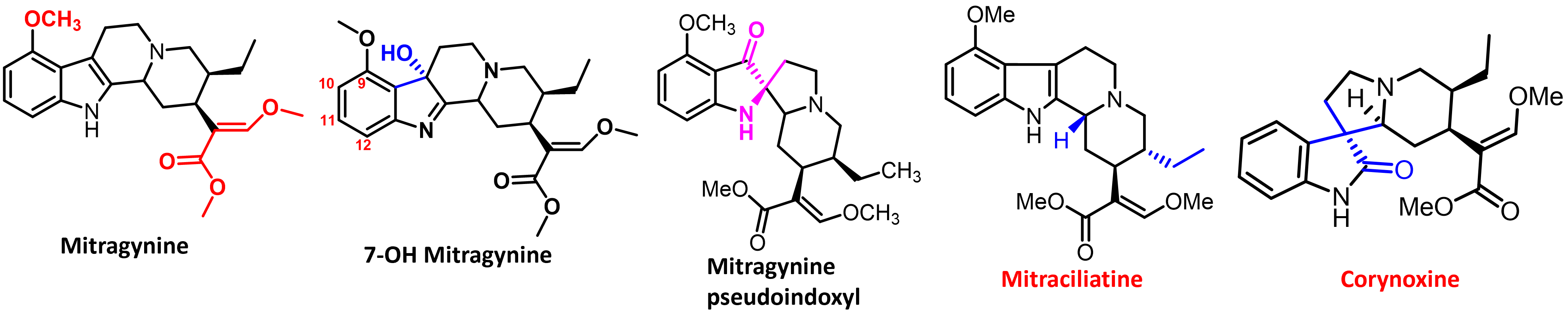
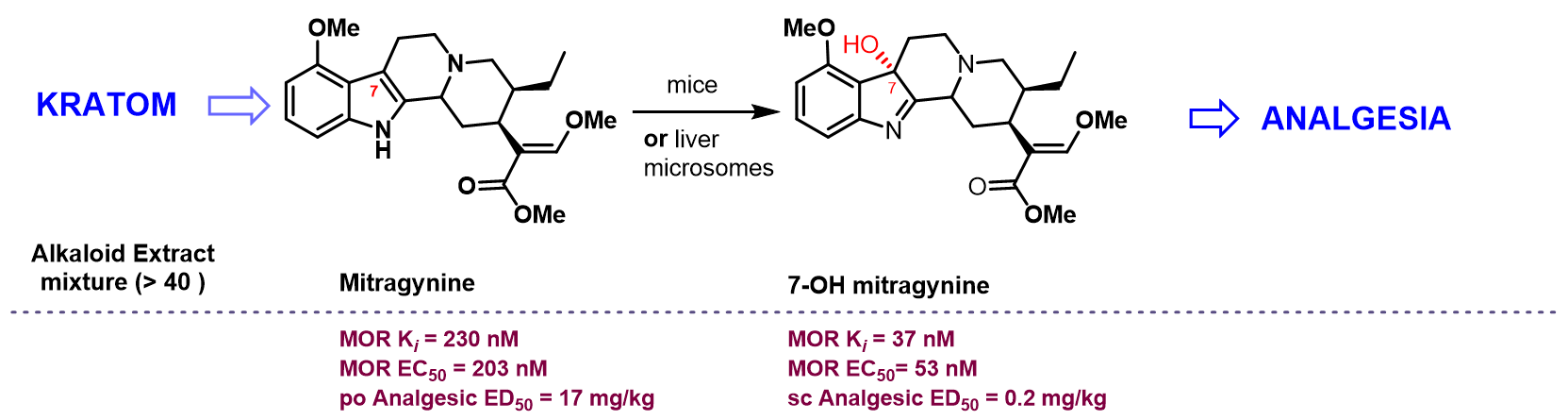
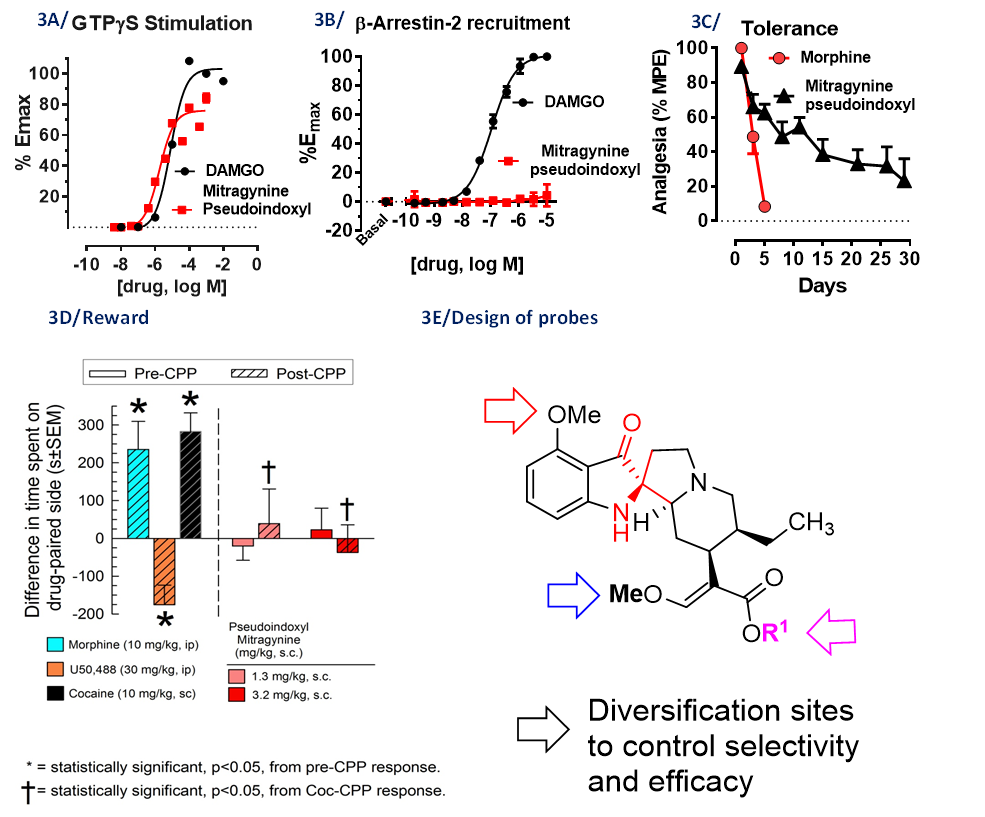
Mitragyna speciosa plant also known, as kratom is a rich source of natural products with opioid activity. Kratom leaves have been consumed by Malayans of South-East Asia for centuries and is recreationally used by 2-3 million Americans. Nearly 40 alkaloids are present in kratom with mitragynine (1-2%) being the major alkaloid (Fig. 1), which show opioid activity. Another semi-synthetic alkaloid derived from mitragynine named mitragynine pseudoindoxyl in this series has potent opioid mediated analgesia while showing opioid functional selectivity in vivo (Fig. 3A-E). We recently showed the analgesic actions of kratom’s major alkaloid mitragynine can be explained by its metabolic conversion to 7OH mitragynine (Fig. 2). The molecular mechanisms of how kratom alkaloids exhibit their biological activity (analgesia and/or abuse) is still unknown. Kratom alkaloids (synthetic and semi-synthetics) thereby offer exciting opportunities to discover probes to understand opioid action, novel opioid drug targets and drugs for treatment of both pain and substance abuse disorders. We are interested in the structure activity relationships on all three templates-mitragynine, 7OH mitragynine and mitragynine pseudoindoxyl. Newer molecules in this series of interest include mitraciliatine, corynoxine and a semi-synthetic named SC13.
J Med Chem 2016, 59(18):8381-97
Nature 2018, 553(7688):286-288
ACS Central Sci 2019, 5(6), 992-1001.
J Med Chem 2021, 64(18),13873-13892
ACS Chem Neurosci 2021 ,12(14), 2661-2678
We solved the cryoEM structures of kratom metabolite, mitragynine pseudoindoxyl (MP) along with a high efficacy fenatnyl analog (lofentanil, LFT). Structures elucidate the role of a novel subpocket sp2 labeled by MP in controlling its efficacy at G-protein and arrestin signaling pathways.
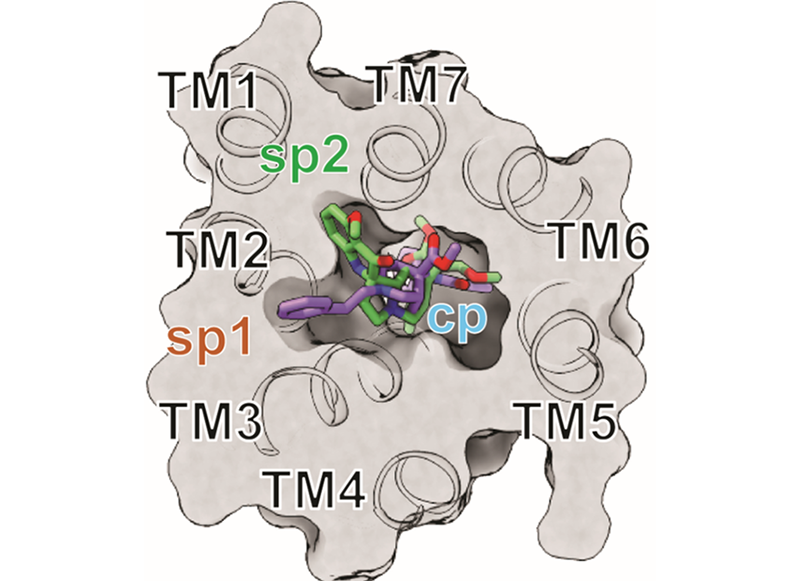
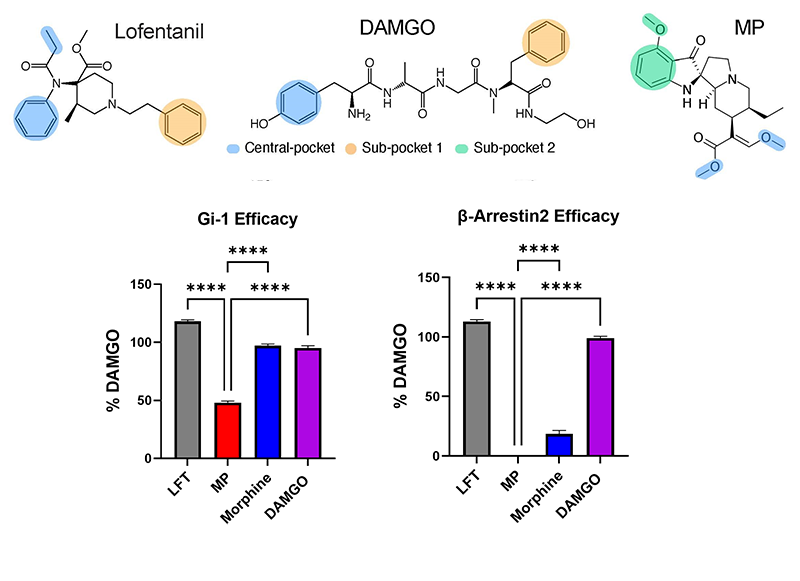
Synthesis of opioid probes which selectively target G-protein biased signaling pathways, βarrestin-2 biased signaling pathways and/or show limited efficacy are useful molecular tools to understand opioid function. Our goal is to identify functional hot spots in the receptor responsible for recruitment of βarrestin-2 and/or control receptor efficacy. We will utilize computational chemistry, chemical synthesis, structure based design, cryoEM and site-directed mutagenesis to identify amino residues and/or transmembrane pockets in the receptor which dictate engagement with βarrestin-2 and/or G-protein efficacy. Lead ligands identified will then be characterized in vivo for their physiological responses like analgesia, locomotor effect, respiratory depression and conditional place preference.
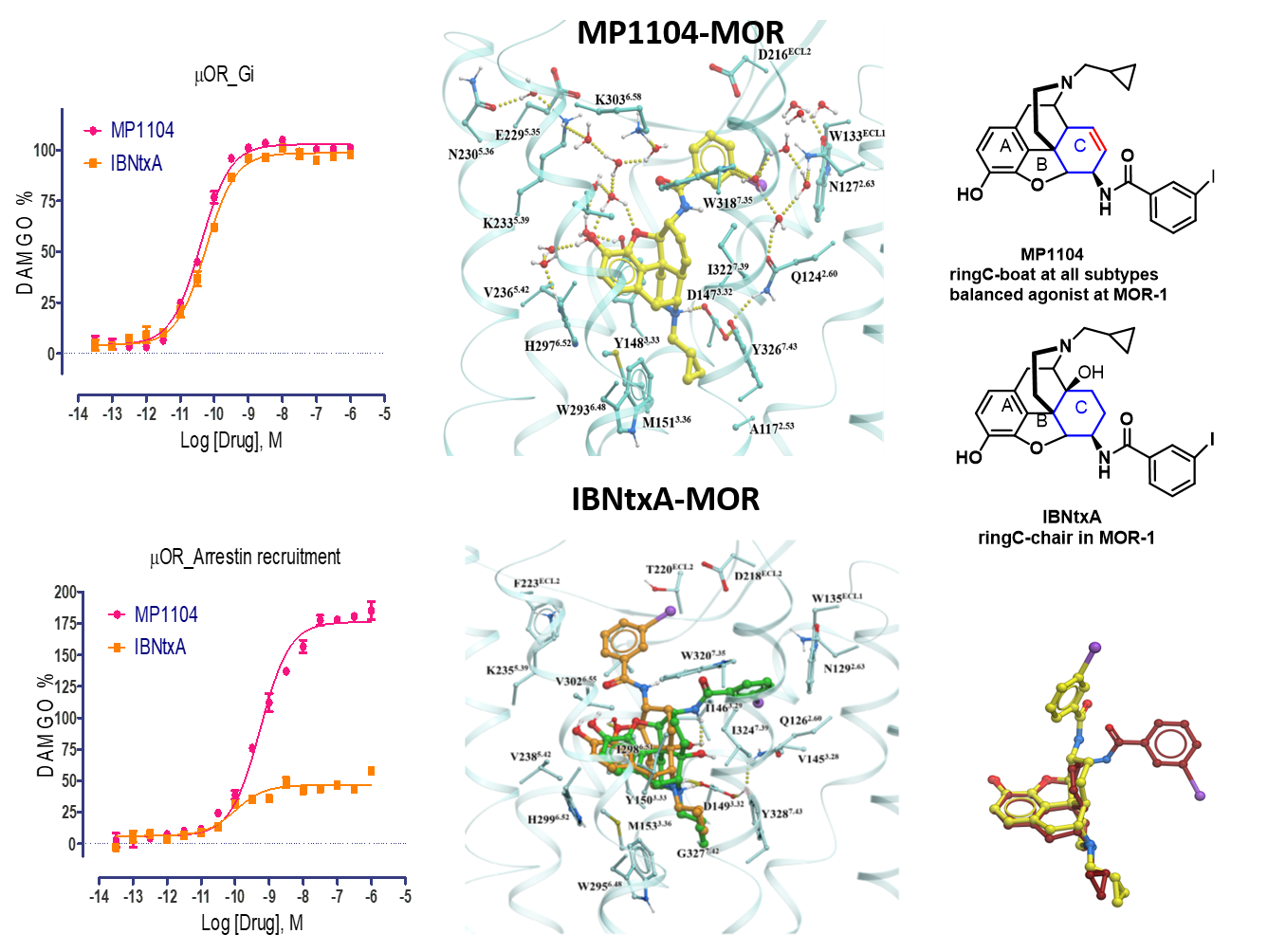
J Med Chem 2021,64(18),13873-13892.
Elife 2021;10:e56519
Molecules 2020,25(18),4257.
Cell 2018, 172(1-2):55-67.
Neuropharmacology 2019, pii: S0028-3908(18)30757-3
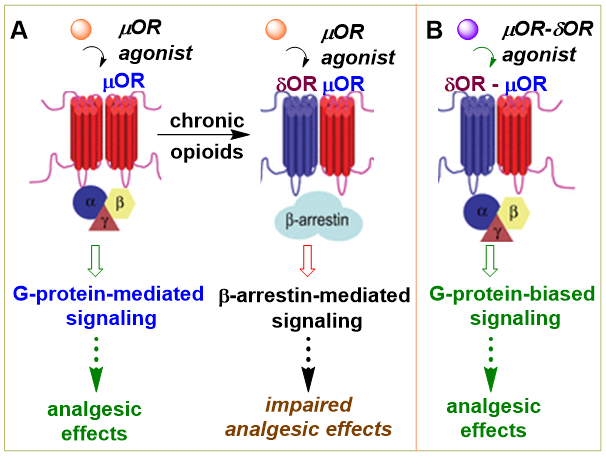
It has been shown that upon activation, the opioid receptors form physiologically relevant dimers which are necessary for maturation, trafficking and downstream signaling. A previous study identified CYM51010 as a small molecule probe that selectively activates μOR-δOR heteromers and exhibits antinociception similar to morphine with reduced antinociceptive tolerance (Gomes et al, PNAS 2013). Since CYM51010 exhibits conditional place preference and respiratory depression in mice, possibly because it retains a moderate efficacy at homomeric μOR in functional assays and is still β arrestin-2 biased, ligands exclusively selective for μOR-δOR heteromers are needed. We are working on carfentanyl amide based opioid templates as novel G-protein biased modulators of μOR-δOR heteromers with a greater selectivity over the homomeric μOR and δOR (when compared to that of CYM51010). An example of this is MP135 (Abdelfattah et al, J Med Chem 2020) we published recently with the Devi group from Mount Sinai
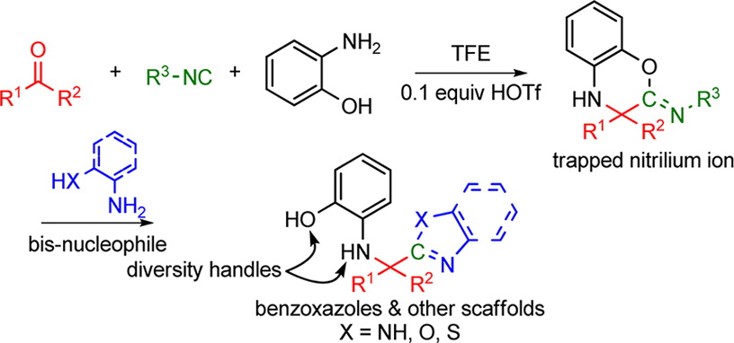
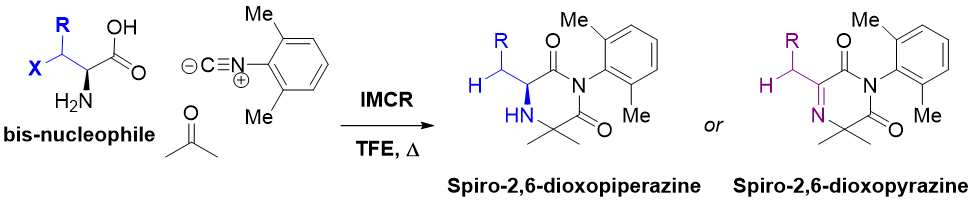
Multicomponent reactions (MCRs) are convergent reactions, in which three or more starting materials react to form a single product, where all or most of the atoms contribute to the newly formed product. The extreme variety of products that can be achieved through the reaction of readily available reagents as well as their one-pot nature and high atom efficiency make MCRs suitable for the synthesis of highly diverse drug-like libraries. We have utilized the isocyanide based Ugi MCR to access diverse heterocycles with activity at CNS targets.

HPLC

Triple Quad LC-MS-MS

LC-MS

Lyophilizer

Plate Reader

HPLC Prep

Tail Flick

Tissue Culture Hood
University of Florida
Jay McLaughlin, PhD
Mount Sinai School of Medicine
Marta Filizola, PhD
Lakshmi Devi, PhD
Stanford University
Ron Dror, PhD
Brian Kobilka, MD
Georgious Skiniotis, PhD
Columbia University
Jonathan Javitch, PhD
Dalibor Sames, PhD
Victoria University, NZ
Bronwyn Kivell PhD
Purdue University
Richard Van Rijn, PhD
Bridge Institute, USC
Vsevolod Katritch, PhD
UNC
Bryan Roth, MD PhD
Washington University
Jose-Moron-Conception, PhD
Robert Gereau, PhD
Tao Che, PhD
Baron Chanda, PhD



